Atsena’s AAV.SPR capsid spreads laterally well beyond the margins of the subretinal injection bleb.
One of Atsena’s novel spreading capsids, AAV.SPR, is being utilized in both the company’s X-linked retinoschisis (XLRS) program and USH1B program. Development of AAV.SPR stemmed from the need to achieve therapeutic levels of gene expression in photoreceptors of the central retina while avoiding the surgical risks of foveal detachment.
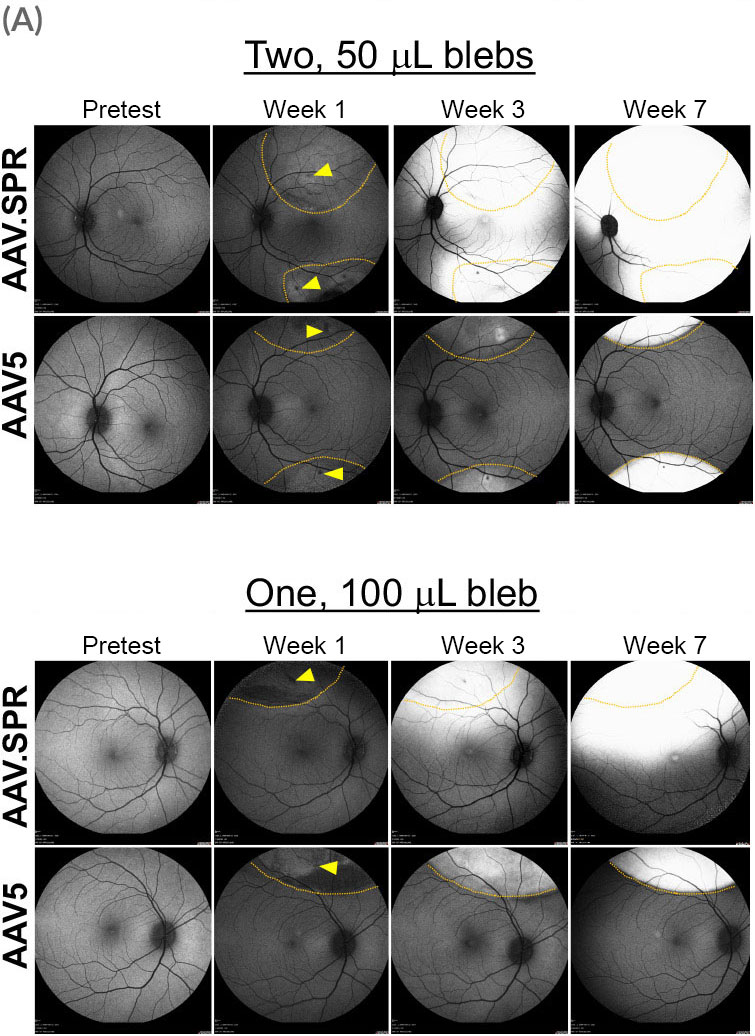
A preclinical study demonstrated that AAV.SPR exhibits enhanced lateral spread in subretinally injected macaques
AAV.SPR containing either myc-tagged human RS1 [AAV.SPR-RS1(myc)] or green fluorescent protein (AAV.SPR-GFP), both driven by a photoreceptor specific promoter were co-delivered at a concentration of 6.6e11 vg/mL. AAV5 vectors containing identical constructs were used as a control.
Eyes received either a single 100 µL bleb placed superior to the macula, or two 50 µL blebs placed superior and inferior to the macula (retinotomy sites delineated by yellow arrowheads). The location of injection blebs relative to the macula and GFP fluorescence over time were captured by confocal scanning laser ophthalmoscopy (cSLO). Borders of original blebs on day-of-dosing were confirmed by OCT and are outlined in yellow. AAV.SPR-mediated GFP expression was seen well beyond the margins of the injection blebs and expression increased over time. Foveal transduction was achieved following placement of either one or two injection blebs. In contrast, AAV5-mediated GFP remained confined to margins of the original bleb, and no foveal transduction was achieved.
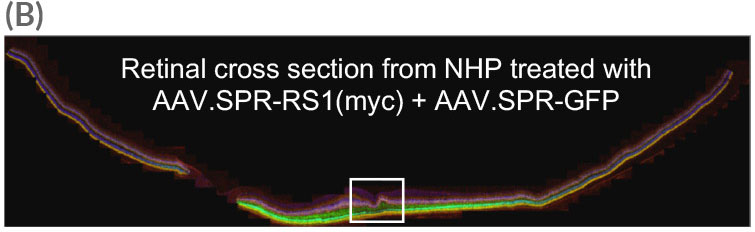
Widefield image of retinal cross section (taken from AAV.SPR treated eye in A) reveals widespread AAV.SPR-mediated GFP and myc expression. White box in (B) is magnified in (C).
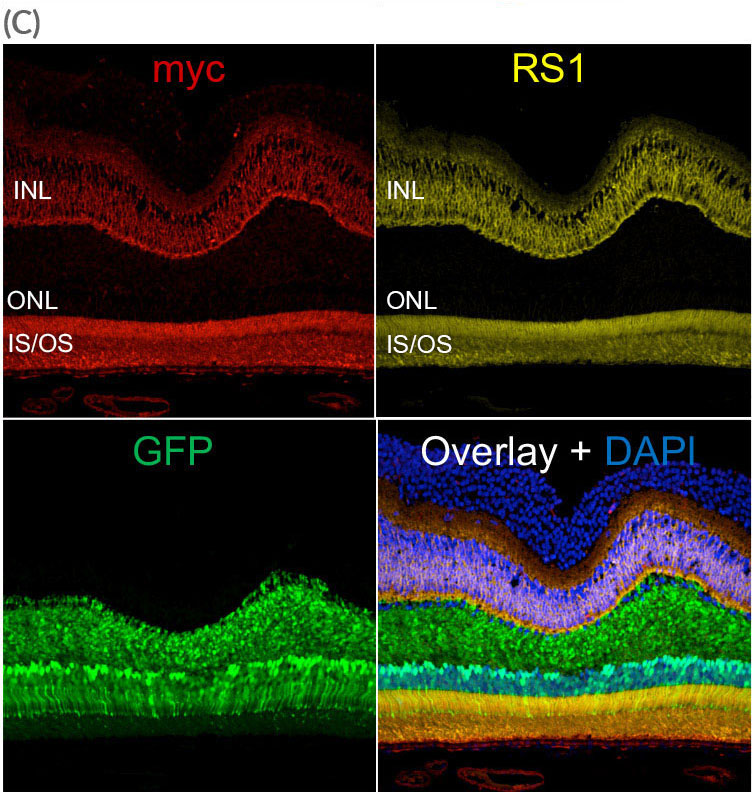
AAV.SPR mediated GFP expression was found in the majority of foveal cones despite this region remaining fully attached during surgery. AAV.SPR-mediated myc (RS1) expression (red) localized properly to photoreceptors and the INL, and colocalized with endogenous RS1 expression (yellow).
IS/OS = inner segments/outer segments of photoreceptors, ONL- outer nuclear layer, INL- inner nuclear layer
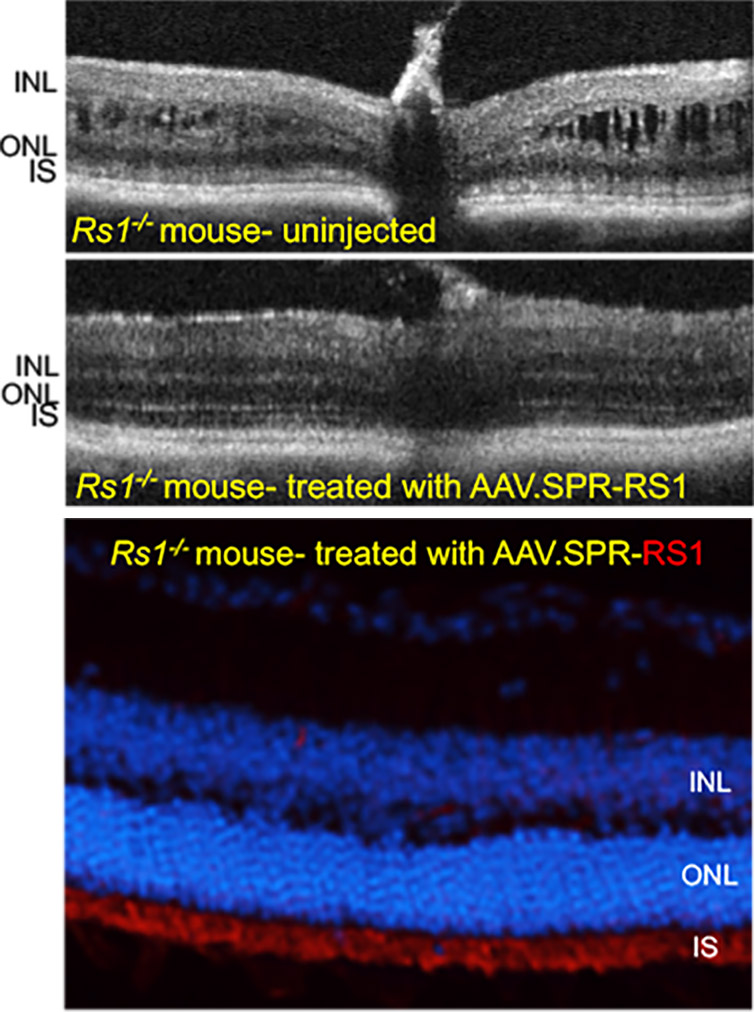
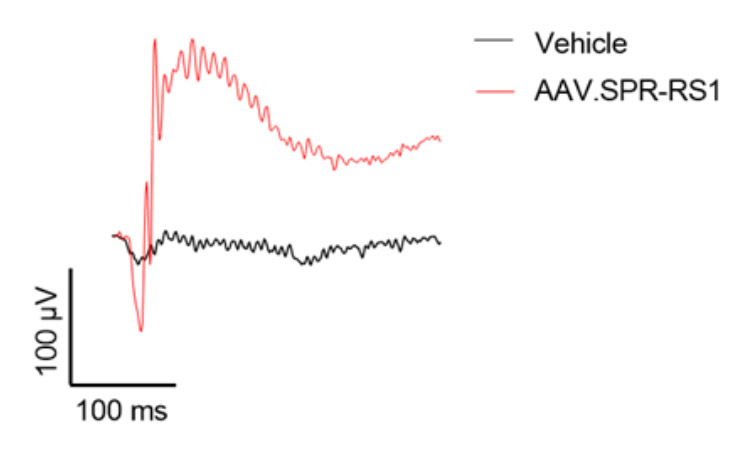
AAV.SPR restores retinal structure and function over the long term to a mouse model of XLRS
Subretinal injection of AAV.SPR-RS1 stably restored retinal structure (completely resolved schisis cavities) and function in Rs1-/- mice as assessed by optical coherence tomography (OCT) and electroretinography (ERG), respectively. AAV.SPR-mediated RS1 expression localized to photoreceptor inner segments (its proposed site of function) of treated eyes. Long term phenotypic rescue of the Rs1-/- mouse (for at least 6 months) further supports use of AAV.SPR to treat vision loss safely and effectively in XLRS patients.
